Navigating Eroom’s Law in Drug Development with AI: A Critical Perspective
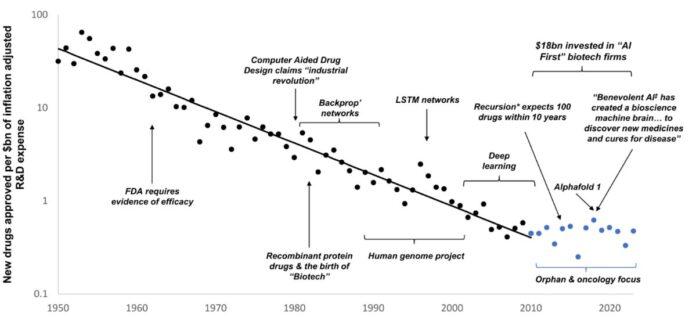
In the realm of drug development, Eroom’s Law reveals a troubling trend—new drug approvals have drastically dwindled despite advances in biomedical science. Here’s what you need to know:
-
The Efficiency Paradox: The number of drugs approved per billion dollars of R&D has halved every nine years, leading to a crisis in pharmaceutical innovation.
-
AI’s Role: While AI promises to solve this problem, the risk lies in focusing on quantity over quality. Simply generating hypotheses won’t overcome the need for rigorous, human-derived data.
-
Cautionary Tales: Rapid advancements in technology can lead to misguided optimism, neglecting critical regulatory reforms necessary for meaningful progress.
-
A Shift is Needed: To reverse Eroom’s Law, embracing human-centric data and streamlining clinical trials is essential. AI must be combined with these approaches to ensure that breakthroughs translate into real-world benefits.
Let’s rethink how we leverage AI in drug development. Share your insights, and let’s drive this conversation forward!